Sawbones and Numalogics Launch Finite Element Analysis Virtual Models
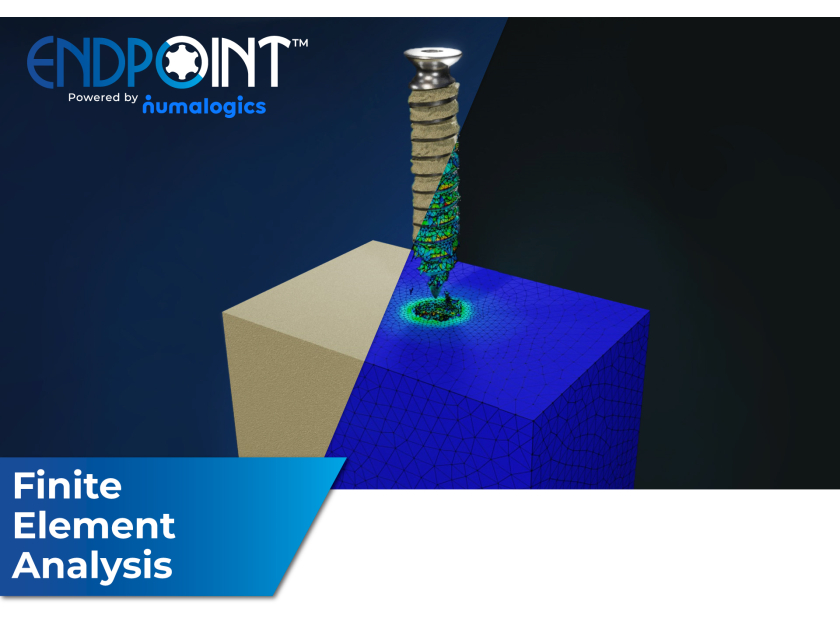
Pacific Research Laboratories, Inc., D.B.A. Sawbones®, and Numalogics Inc., are pleased to announce the launch of a web application that allows orthopedic implant manufacturers to test devices on industry standard Sawbones® bone surrogate (polyurethane foam), completely in a virtual environment. With the new web application, named “ENDPOINT™”, engineers can now easily test orthopedic screw designs using the latest computational modeling and simulation techniques and obtain results within days. With this new testing method, device prototyping can be reduced, or even eliminated, saving company time and money.
Customers can select the type of mechanical screw test to be performed, and, using a secure method, screw design drawings are uploaded into the cloud. An automated application then performs the simulation of the selected test, and results are provided back to the customer within 2 days*. Data security and confidentiality are maintained throughout the process.
Amy Posch, M.S. Design Engineer-Biomechanical at Sawbones®, says: “We are happy to offer a CM&S tool that demonstrates a high level of credibility for axial screw insertion and pullout testing. Transparency in the models’ performance was important to convey and we are impressed with its verification and validation work. We look forward to expanding the biomechanical material suite available within this platform.”
Sawbones® and Numalogics have worked collectively to develop robust virtual models of Sawbones® polyurethane foam, the industry standard bone surrogate material for orthopedic implant testing, and to validate the existing virtual screw tests.
ENDPOINT™ is now available!
White papers detailing the validation results available here!
*some conditions apply

Obtain results in three easy steps:
1 - Place your order
2 - Securely upload your data
3 - Download your results
Results will include:
Test Report (.pdf file)
Animation (.mp4 file)
Raw Data (.csv file)