First-Ever FDA Qualification of an Orthopedic Device Mechanical Test Under MDDT Program
In a groundbreaking advancement for the orthopedic device industry, Sawbones and Numalogics, Inc. are proud to announce that their jointly developed Finite Element Analysis (FEA) model for orthopedic screw pullout testing has received formal qualification from the United States Food and Drug Administration (FDA) through its Medical Device Development Tools (MDDT) program. This achievement marks the first-ever FDA qualification of a mechanical test for an orthopedic device, setting a new precedent for virtual testing in regulatory science.
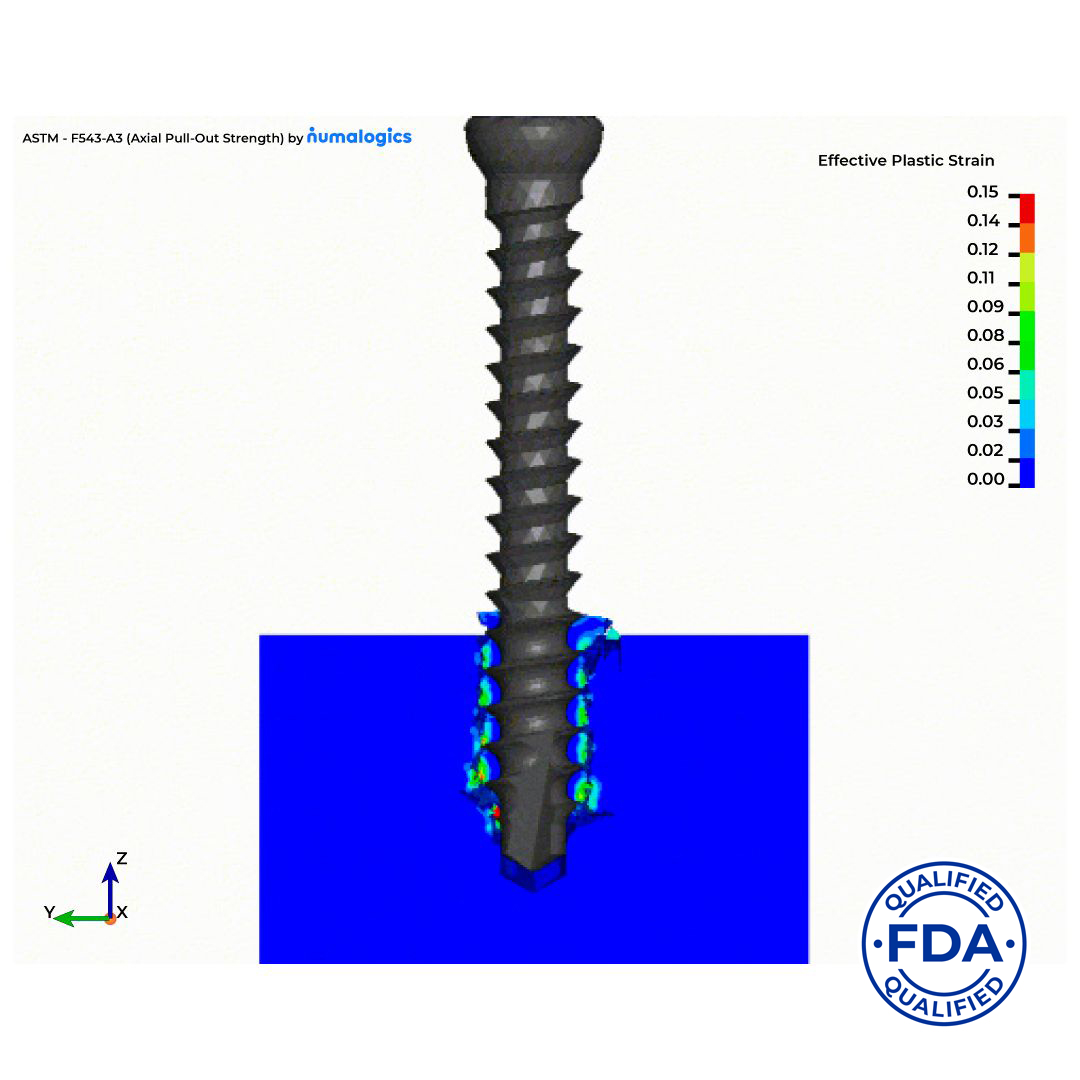
The qualified tool simulates screw pullout behavior in accordance with ASTM F543, a widely accepted standard for evaluating the mechanical performance of orthopedic screws. Built on Sawbones’ industry-standard synthetic bone materials and powered by Numalogics’ advanced FEA modeling technology, the model enables accurate, reproducible virtual testing, reducing reliance on time-consuming and costly physical bench tests. This validated virtual method allows orthopedic device manufacturers to replace or supplement physical testing for both product design optimization and regulatory submissions.
“This landmark FDA qualification highlights and elevates the role of standardized bone simulation in medical device development and regulatory science,” said Amy Posch, M.S. Design Engineer-Biomechanical at Sawbones®, “Together with Numalogics, we’ve delivered a tool that is both scientifically rigorous and regulatory-ready.”
By qualifying this virtual test, the FDA has recognized its ability to produce trustworthy and standardized evidence as a surrogate to physical testing using ASTM F543 screw pullout testing protocols, and synthetic bone materials manufactured per ASTM F1839.
“This isn’t just a technical validation — it’s a shift in how the orthopedic industry can approach design and regulatory submissions,” said Eric Gaudreau, President of Numalogics. “With this FDA-qualified tool, companies can now leverage digital tools to reduce risk, accelerate development, and improve consistency.”
If you’d like help incorporating this tool into your FEA workflow or understanding how it could support your current device pipeline,
we’re happy to provide additional technical details.